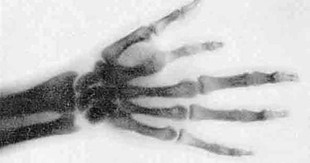
Home / Protected: Why don’t we see in X-rays?
Categories
Tags
adult learner
algorithm
algorithms
bearings
buildings
cancer
careers advisor
Christmas
coding
Competition
Competition winners
coordinates
cymru
data science
environment
Flexagon
fractions
games
game theory
gcse
health
ks4
machine learning
modelling
music
National 5
navigation
pi
profile
Pythagoras
python
scale
space
square root
statistics
teacher
teachers
Weather Forecasting
whales
Archives
- April 2024
- March 2024
- February 2024
- January 2024
- December 2023
- October 2023
- May 2023
- April 2023
- March 2023
- February 2023
- December 2022
- November 2022
- August 2022
- May 2022
- April 2022
- March 2022
- February 2022
- December 2021
- November 2021
- October 2021
- September 2021
- June 2021
- May 2021
- April 2021
- March 2021
- February 2021
- January 2021
- December 2020
- November 2020
- October 2019
- July 2019
- April 2019
- March 2019
- February 2019
- January 2019
- December 2018
- October 2018
- September 2018
- August 2018
- July 2018
- June 2018
- April 2018
- March 2018
- February 2018
- January 2018
- November 2017
- October 2017
- September 2017
- August 2017
- July 2017
- April 2017
- March 2017
- February 2017
- January 2017
- December 2016
- November 2016
- October 2016
- September 2016
- August 2016
- July 2016
- June 2016
- April 2016
- March 2016
- February 2016
- January 2016
- December 2015
- November 2015
- October 2015
- September 2015
- August 2015
- July 2015
- June 2015
- May 2015
- April 2015
- March 2015
- February 2015
- January 2015
- November 2014
- September 2014
- June 2014
- May 2014
- March 2014
- February 2014
- November 2013
- August 2013
- June 2013